Mastering the daily: How a Daily Management System drives excellence in pharma operations
Key Highlights
- A DMS provides a structured framework for managing daily operations, focusing on visual management, structured meetings, and problem-solving methods.
- Digital DMS solutions integrate real-time data and analytics, transforming manual processes into dynamic, data-driven workflows that enhance visibility and responsiveness.
- In pharma, DMS helps manage complex, regulated processes by ensuring consistency, traceability, and early detection of deviations to maintain product quality and compliance.
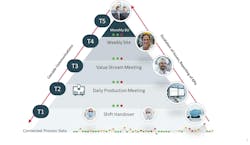
- Effective communication is achieved through a tiered, visual information flow that aligns teams across shifts and functions, fostering coordination and accountability.
- Future developments in DMS include AI-powered insights and decision-enablement tools that support faster learning, adaptation, and continuous improvement.
In pharmaceutical processing, every shift counts. Each handover, decision and data point contributes to product quality and operational stability. Yet in many facilities, daily management still relies on fragmented routines — isolated meetings, inconsistent communication and limited visibility across teams. A Daily Management System (DMS) brings structure to that complexity. By connecting people and processes through clear, visual routines, a DMS helps teams stay aligned and focused on continuous improvement.
Q: For readers unfamiliar with the concept, what exactly is a DMS?
A: A DMS is a structured framework for managing and improving operations on a day-to-day basis. It provides the data, discipline and structure that keep teams focused, accountable and aligned around shared goals.
At its simplest, a DMS defines how performance is reviewed, how information flows between teams and how problems are identified and resolved. While every organization adapts the concept to its own needs, most DMS frameworks share a common set of elements:
- Visual management: Clear, real-time dashboards or displays showing key performance indicators, issues and progress toward goals.
- Structured daily meetings: Short, focused discussions to review performance, highlight deviations and align on priorities.
- Standardized workflows: Defined processes for logging events, documenting actions and ensuring consistent execution across shifts.
- Structured problem solving: Methods such as PDCA or root cause analysis for resolving issues.
- Escalation paths: Clear channels for moving unresolved issues to the right level of management for timely decisions.
- Continuous improvement: Mechanisms to capture lessons learned, track corrective actions and sustain improvements over time.
Together, these elements create a daily rhythm of communication and accountability. They ensure that every shift contributes meaningfully to long-term objectives, turning routine operations into a foundation for continuous improvement.
Q: The DMS concept originated in Lean Manufacturing. How has it evolved for today’s digital environment?
A: The DMS has its roots in the Toyota Production System, where principles such as Gemba and Kaizen defined continuous improvement. Traditionally, DMS practices were manual, relying on whiteboards and physical meetings. Today, with digital platforms that are intelligent operations platforms, we have transformed these Lean principles into dynamic, data-driven processes.
Digital DMS solutions integrate real-time data, automate workflows and offer advanced analytics that provide insights impossible to achieve with manual systems alone. They not only improve visibility and responsiveness but also provide a foundation for continuous learning and improvement across the organization.
Q: Why is DMS so essential for the pharmaceutical industry in particular?
A: Pharmaceutical processing operates under some of the most demanding conditions of any industry. Processes are highly regulated, product quality must be proven and documented at every step, and even minor deviations can trigger costly delays, rework or compliance findings. The margin for error is vanishingly small.
A DMS helps processors manage that risk. It creates the consistency needed to maintain control in complex, round-the-clock process manufacturing operations. By establishing a structured rhythm of monitoring and communication, it ensures that performance issues, deviations or bottlenecks are detected early, before they affect product quality or regulatory outcomes.
Just as importantly, a DMS reinforces the culture required for compliance. It brings transparency to daily operations, promotes accountability and ensures that information flows seamlessly between shifts and departments. In an environment where documentation, traceability and rapid response are essential, the DMS provides the framework that keeps production both efficient and compliant.
Q: How does a DMS support team alignment and effective communication in pharma operations?
A: In pharmaceutical operations, communication often fragments across shifts and functions. A DMS closes those gaps by creating a clear, repeatable flow of information and a “single source of truth” that is shared across all teams, shifts and management layers. This tiered structure supports short, focused meetings at each level where teams review key metrics, address issues and escalate what cannot be resolved on the spot. This ensures that decisions move quickly to the right level of accountability.
Visual dashboards complement this process by making performance transparent. When everyone sees the same data — production results, quality trends or deviations — alignment becomes natural. The result is a connected operation where information flows up and down the organization with speed and clarity, turning daily communication into a driver of coordination, performance and continuous improvement.
Q: How do you see DMS evolving in the coming years?
A: The fundamentals of daily management — communication, structure and accountability — will not change. What is evolving is the level of intelligence and visibility that supports them.
We are moving from systems that simply display information to ones that actively interpret it. Visual intelligence tools are beginning to highlight trends, predict risks and guide attention to where it is needed most. Artificial intelligence (AI) is accelerating this shift — not just by analyzing data, but increasingly through agentic AI, which can take context-aware, goal-oriented actions. These systems will help teams prioritize issues, suggest responses and even initiate routine follow-ups.
In the future, the DMS will function less as a reporting layer and more as a decision-enablement platform, connecting people with the right context at the right time. The goal is not to automate leadership but to augment it: turning data into shared understanding and helping teams learn, adapt and improve faster. That, ultimately, is what continuous improvement in the digital era looks like.
Q: Where should a pharmaceutical processor start when implementing a DMS?
A: The key is to start simple but start deliberately. A DMS does not have to be rolled out across an entire site on day one. Many organizations begin with a single line, department or pilot area to establish the rhythm of daily reviews, visual performance tracking and clear escalation paths.
The goal is to build consistency first, helping teams experience the benefits of structured communication and transparency in their own area. From there, the framework can scale naturally across the plant. Equally important is leadership engagement. When managers model daily accountability and data-driven decision-making, the system becomes part of the culture rather than a new layer of process. Over time, that consistency turns into a powerful engine for continuous improvement.