The perfect climate for capsule storage
As a dosage form, capsules have been around for a long time. The first recorded patent for gelatine capsule production was in 1834. It would be reasonable to assume that after some 180 years of experience there would be no secrets about how to achieve optimal capsule quality and consistency. While that assumption is mostly true, things have also changed since the first capsules were produced. New technologies have entered the production space, formulations and ingredients have changed to meet market demands, and even the climate itself has changed with seasonal temperature and humidity reaching new levels. One thing that has already changed and will also continue to develop, is the need to produce capsules with consistent quality characteristics, particularly when producing GMP-compliant products. Not surprisingly, humidity control during the drying and storage processes plays a large role in achieving quality targets.
Capsule materials and characteristics
The most common material used for capsule production is gelatine. Regardless of the source and type of gelatine, the actual physical characteristics of different types of gelatine are similar from a drying and storage perspective. For example, the melting point of all types of gelatine is around 30°C to 32°C. This presents challenges for production in climates where temperatures regularly exceed this. As early as 1913, Eli Lilly first used air conditioning to allow production at times when the air temperature was above 30°C — prior to this innovation production stopped, as the gelatine would not set. Since then, some form of air treatment has become the norm in production areas.
After gelatine, hypromellose (HPMC) is also used to produce capsules. Its plant-based ingredients pose few dietary objections than animal-based gelatines, and it is well-suited to fillings that are sensitive to moisture or that may not work well with the residual moisture found in gelatine capsules (e.g. powders used in inhalers).
Moisture content of finished capsules
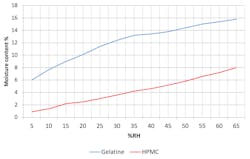
Empty gelatine capsules have a moisture content between 13% and 16% — they will become brittle if the moisture content falls below this limit, and will soften if it increases above it. Empty HPMC capsules have a moisture content of 3% to 6%. These capsules can be dried down to less than 1% moisture without losing their mechanical strength and becoming brittle. Regardless of the material used, this fluctuation can lead to some degree of compromised capsule strength and overall quality, so it needs to be considered from the very outset of production.
Moisture content of gelatine and HPMC capsules will vary depending on the ambient conditions as the moisture content will gradually reach equilibrium with the surrounding relative humidity level. Figure 1 shows the changes that occur after storage for one week at varying humidity levels.
Therefore, humidity must be considered when capsules are stored to ensure the required moisture content is maintained, particularly if water vapor exchange between the capsule and the filling may also contribute to the overall available moisture content.
Packaging and storage — how gelatine reacts to relative humidity
When capsules are packaged, attention should be given to the ambient conditions, as a small quantity of the surrounding air will be sealed in with the product. Depending on the volume of the packing container, this could introduce a quantity of water vapor that may affect the capsules. In general, HPMC capsules are not as vulnerable to this water vapor as gelatine capsules are, so from this point on only gelatine capsules will be considered.
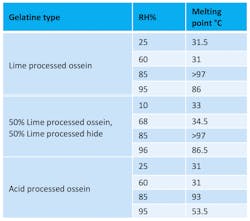
Table 1 shows the effect on the melting point of gelatine films stored at various relative humidity levels after conditioning at 50°C for eight days. The melting point/dissolution temperature of the gelatine film was determined by placing the film in a water bath at 20°C, and the temperature was raised by 1.7°C per minute until the gelatine dissolved. It is interesting to note that the initial melting point of the samples was 32°C, which correlates to the gelatine samples that were stored in low humidity conditions.
At a relative humidity level of 85%, the maximum change in melting point is observed; excessive moisture absorption at higher levels increases the separation between gelatine molecules and makes reformation of bonds more difficult. Increased melting point is a problem, as the gelatine will not dissolve as expected when the patient ingests the capsules.
High relative humidity causes by cross-linking
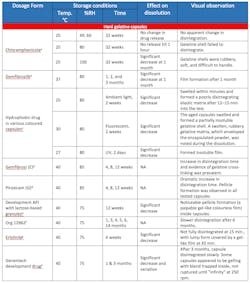
As shown in the data presented above, long-term storage of gelatine capsules at raised temperature and humidity levels (e.g. 40°C, 75% relative humidity for six months) changes the gelatine composition as cross-linking of gelatine molecules occurs. This reduces the solubility of the capsules — in the worst case, rendering capsules practically insoluble. Table 2 is a collection of observations from various manufacturers that show instances of gelatine cross-linking in actual products.
In addition to this, variations in relative humidity outside of the recommended levels can cause hydrothermal contraction in gelatine films. For example, a 0.6mm-thick gelatine film that is exposed to 80% relative humidity and 21°C reduces in size by 2.4% in relation to the overall film length1. Changes in the size of the capsules may lead to:
- Changes in the capsule dimensions and tolerances for closing
- Contents leaking
- Changes in physical appearance
- Decreased patient compliance if they feel the product is no longer stable
Correct storage and packaging are important to achieve maximum product shelf life and achieve target quality levels. As mentioned earlier, finished gelatine capsules have a 13% to 16% moisture content, so storing in the range of 35% to 65% relative humidity and 15 to 25°C (59 to 77°F) will maintain this.
By comparison, HPMC capsules contain less water than gelatine alternatives, and changes in moisture content has less effect on capsule dimensions.
In closing
Relative humidity can impact the quality of both gelatine and, to a lesser degree, HPMC capsules during production and storage. If the air is too dry, the gelatine capsules become brittle, particularly if they are kept in their unfilled, open state.
More seriously, the potential for gelatine to undergo cross-linking and hydrothermal contraction increases in relation to the relative humidity level, which leads to diminished product quality.
On the other hand, when the relative humidity level is too low, static electricity can build up in the production area. This leads to capsules sticking to each other or being difficult to package properly as the capsules are attracted to the plastics used on the production and packaging lines.
With accurate control of relative humidity levels, these problems can be avoided and product quality will benefit as a result.
Martin Ginty is Global Pharmaceutical Industry Manager for Munters, a leader in energy efficient air treatment solutions based on expertise in humidity and climate control technologies. Ginty joined Munters in 2014 and is currently responsible for its overall product and solution offering for pharmaceutical applications worldwide. He has more than 20 years of experience in implementing national and international level automation and HVAC projects, and can be reached at [email protected].
References
- Figure 1: Capsugel Library - Performance Qualification of a New Hypromellose Capsule
- Table 1: Jopling, 1956 – Society of Chemical Industry
- Table 2: http://www.dissolutiontech.com/issues/201708/DT201708_A01.pdf
- 1 Calhoun and Leister, 1959