Transform quality deviation investigations with AI-driven advanced analytics
Key Highlights
- AI platforms enable real-time monitoring and automatic deviation detection, reducing investigation time and preventing major quality issues.
- No-code/low-code tools empower SMEs to perform in-depth root cause analysis without advanced data science expertise, streamlining workflows.
- Automated reporting ensures consistent, accurate documentation that meets regulatory standards, saving time and reducing audit risks.
- Consolidated dashboards and contextual data improve visibility across departments, facilitating quicker decision-making and collaboration.
- Case studies demonstrate how AI integration transforms manual processes into efficient, automated workflows, enhancing compliance and operational efficiency.
Investigating quality deviations is an essential task in process manufacturing, but it is not without complexity. Many facilities struggle with inefficiencies during these procedures, causing delays and operational strain.
This article identifies key challenges that organizations face during quality deviation investigations, then describes how artificial intelligence (AI) and advanced analytics platforms help plant personnel streamline these workflows.
Traditional challenges
The five primary challenges confronting teams historically include:
- Resource allocation inefficiencies: Operations teams are often pulled from critical, value-added activities to assist with manual data gathering and reporting during the investigation, while quality teams can be overwhelmed by repetitive documentation tasks and paperwork. Inefficiencies in the workflow not only slow down investigations, but also increase the risk of prolonged production delays, along with revenue losses as a result.
- Manual and time-intensive procedures: Investigations require significant time to manually collect data from various fragmented systems — e.g., process data from historians, batch context information from manufacturing execution systems (MES) and quality data from laboratory information management systems (LIMS). Aligning and cross-referencing information among these disparate systems is cumbersome and often requires coordination among multiple teams. The time spent manually collecting and reconciling data can also increase required investigation time and potentially introduce production downtime.
- Limited real-time visibility and contextualization: The lack of real-time visibility of critical process data makes it difficult to efficiently identify a deviation’s root-cause. This challenge is compounded by missing contextual information — such as batch number, product and key phases of the process — and siloed data across departments. This can cause prolonged process disruptions due to inefficient root-cause identification and delayed release of products held in quarantine.
- Reactive culture: In many process manufacturing environments, deviations are often identified only after the fact, rather than through predictive or preventative real-time monitoring. The reactive culture results in frequent production disruptions, causing significant delays in product timelines. Repeated similar deviations also suggest an inefficiency of leveraging historic events and knowledge which can prevent long-term quality and efficiency improvements.
- Documentation burden: Certain industries in particular — such as pharmaceutical, food and beverage, and healthcare manufacturing — require stringent documentation for regulatory compliance. However, the manual and often inconsistent approach to documenting investigations increases the likelihood of gaps in the audit trail. Additionally, inconsistent investigation methodologies across multiple teams create inefficiencies in documentation, complicating the review process. The manual documentation burden places significant strain on plant personnel, increasing the time spent on non-value-added tasks.
Transforming quality deviation workflows using AI and advanced analytics platforms
AI-driven analytics platforms can address the challenges of quality deviation investigations by enabling real-time data access, intelligent investigation and automated reporting. These solutions provide significant business impact in improved quality outcomes by decreasing time to deviation resolution, enabling comprehensive knowledge capture and increasing report accuracy and efficiency.
Some of the most notable benefits these platforms provide include:
- Real-time detection and flagging: Advanced analytics platforms, such as Seeq, integrate multiple data sources, aligning timestamps and key process parameters to create a unified view of the manufacturing process. Operators and subject matter experts (SMEs) can monitor these parameters in real-time through dashboards, with the system capable of automatically flagging deviations, which eliminates detection delays and expedites investigation. This reduces the time between when issues occur and corrective or mitigative actions are performed, providing quicker resolution, and even potentially preventing major quality deviations in the future.
- Intelligent investigation: When a deviation is flagged for investigation, engineers can apply powerful no-code/low-code analytics tools for deeper analysis. This empowers SMEs to quickly identify trends, correlations and potential root causes without the need for advanced data science degrees, enabling more efficient investigations and providing more time to spend on value-added process understanding and troubleshooting tasks. It is best practice to keep all investigative information in a single location for collaboration and root cause analysis, which can later be leveraged by AI in reporting and later investigations, such as by using Seeq Vantage (see case study that follows).
- AI-powered reporting: AI can be leveraged to generate investigation reports automatically, capturing all necessary details to meet regulatory standards, in addition to leveraging captured historic event context for additional insight. These reports are easily accessible to leadership teams, eliminating the hours typically spent on manual documentation. Automating the reporting process significantly reduces the time spent on administrative tasks. Additionally, automated reports ensure consistent and accurate documentation that can reviewed by relevant personnel, reducing the risk of non-compliance.
- Secure review and compliance: Leading advanced analytics platforms provide locking and versioning functionality to ensure changes to analyses are fully documented and auditable to support and maintain industry compliance. This minimizes the risk of audit failures and non-conformance issues.
Case study: Streamlining a quality deviation workflow using Seeq
A prominent pharmaceutical manufacturer implemented Seeq, an AI-equipped advanced analytics platform, to transform its manual deviation investigations into integrated, automated and efficient workflows, employing four key steps (Figure 1).
Step 1: Real-time monitoring in Seeq Organizer
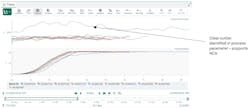
The company began by setting up operational monitoring in a consolidated Seeq Organizer dashboard. This provided real-time access to critical process data, including critical process parameters (CPPs) and performance trends.
In the example used throughout this case study, an operator noticed a deviation in Reactor 1. The volume signal was outside of normal limits, clearly flagged in the visual display (Figure 2).
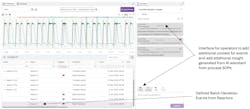
Step 2: Triage and contextualization in Seeq Vantage
Next, the operator opened Seeq Vantage, which enables enterprise-scale monitoring and contextualization. Within Vantage, the operator found the flagged deviation event, categorized it added contextual information, and escalated it to the area’s process engineer (Figure 3).
Using integrated AI connected to company documentation and standard operating procedures (SOPs), the operator gathered additional insights regarding possible causes or corrective actions for the deviation.
Step 3: Root cause analysis in Seeq Workbench
The flagged event appeared in the engineering team’s tailored Vantage Room, which was similar to the interface shown in Figure 2. Rooms can be customized by user or group to display only specific events of interest, and events can be organized according to user requirements. In this example, events are grouped by asset.
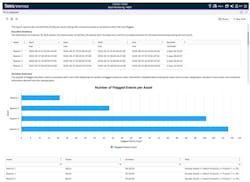
The engineering team began its investigation of the deviation event using Seeq Workbench. Leveraging the clean, aligned data from multiple sources and a suite of no-code/low-code analytical tools, they compared the current batch behaviors to historical batches and identified an abnormal trend in one of the control signals, indicating a likely root cause (Figure 4).
Step 4: Reporting and knowledge capture
The team then added their findings to Vantage, marking and escalating the event for review by quality and production managers. Leveraging its AI capabilities, the platform automatically created a full investigation report, integrating data with the added context (Figure 5).
Adding business value and saving time
The pharmaceutical case study exemplifies how AI and advanced analytics can dramatically transform investigation workflows. By providing operations, engineering and quality personnel with real-time access to consolidated process visuals and trend data, teams can triage, categorize and deeply examine critical events at scale. Furthermore, the full process context of stored information simplifies document investigations and facilitates close collaboration.
AI support also accelerates insight generation, saving SMEs significant amounts of time previously required to manually create compliance documentation and reports. Advanced analytics platforms capture and retain critical process knowledge from contextualized events and leverage AI to close persistent gaps in process data.
Empowering teams to learn from past deviations and proactively prevent recurrence, AI and advanced analytics tools help businesses accelerate their value-additive efforts by reducing quality risk, strengthening regulatory compliance and optimizing operational efficiency.
About the Author

Asif Hassan
Analytics Engineer at Seeq
Asif Hassan is an Analytics Engineer at Seeq, assisting organizations with uncovering data-driven insights from their processes. With six years of experience in the process engineering industry, he has developed extensive knowledge in product development and manufacturing operations across various sectors, including pharmaceutical, chemical and food manufacturing. Asif holds bachelor’s and master’s degrees in chemical engineering from University of Surrey and Imperial College London, respectively.

Tatum O’Kennedy
Senior Analytics Engineer at Seeq
Tatum O’Kennedy is a Senior Analytics Engineer at Seeq, helping pharmaceutical companies identify key trends in their data. With nine years of experience, she has developed extensive knowledge in formulation development, product development and commercial manufacturing. Tatum holds bachelor’s and master’s degrees in chemical engineering from Northeastern University. She is a member of the International Society for Pharmaceutical Engineering and regularly shares insights on LinkedIn, helping advance the field of data analytics in the pharmaceutical industry.