The close examination of solid-liquid separation processes may not have the glamor of investigating blackmail and burglary or extortion and espionage, yet process engineers can still learn many things from Sherlock Holmes and Dr. John Watson.
The solid-liquid separation challenge
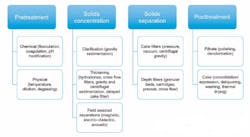
Process engineers must use their own kind of sleuthing in selecting the right type of solid-liquid separating equipment. It’s not an easy task. The wide range of equipment that can be employed and the illogical solutions that are often required can cause confusion. As a further complication, only two basic principles of liquid/solid separating exist, which means equipment that might not be the ideal choice must be made to work, while admittedly sacrificing some efficiency.
Engineers must be particularly careful when working with suppliers that only offer one type of equipment. The solution they are offering may work but not necessarily be the smartest choice. Engineers confronted with a solid-liquid separation challenge must carry out their own basic evaluations of possible or probable solutions.
Several approaches to categorizing solid-liquid separation equipment are available. First, the engineer must decide whether the process is batch or continuous. This is not as simple as it sounds. For example, while a reaction/precipitation process may be batch or continuous, the same choice pertains to the solid-liquid equipment. Engineers must also examine the downstream process and determine whether this process is batch or continuous. Most often, a continuous process is more efficient. However, other process aspects such as time in the reactor, crystal sizing/breakage, solids handling (or alternatively, whether the process can handle concentrated slurry rather than solids), drying time and other parameters must be considered.
Having reviewed the process, the next factor to consider is what type of discharge is required: batch or continuous, dry solids, wet solids, or concentrated slurry. The engineer must know if the solids, liquids or both are considered as the "product," as well as whether the overall process is automated or manual. For instance, are the solids directly discharged to the downstream process or moved by conveyors, totes or intermediate bulk containers? Or are other systems employed?
Read Also: Filtration system yields sweet benefits
Finally, the engineer must examine the amount of solids to be filtered. For example, does the process slurry contain "high solids," which could be up to 50 to 60 percent solids, or low solids, whether in the 2 to 5 percent range or down to trace amounts at the parts per million (ppm) level?
Principles & mechanisms for effective solid-liquid separation
The two basic principles of solid-liquid filtration involve either separating liquids from solids or filtering solids from liquids, using one of two principles:
- That the solids will have a tendency to go one way and liquids the other (separation)
- That use will be made of a hole smaller than the solids to be captured (filtration)
Still, more than 100 different types of equipment are available, many with their own variations.
Focusing on solid-liquid separation, suspended solids are removed from liquid either on the surface (cake filtration) or within the depth of the filter medium. The depth of the filter media can be the filter media itself, the cake or the filter aid. Regardless of the surface of depth filtration, three mechanisms exist for removal: inertial impact, diffusional interception or direct interception.
Particles in a fluid have a mass and velocity and therefore an associated momentum. As the liquid and entrained particles pass through a filter media, the liquid will take the path of least resistance and be diverted around the fiber. Because of their momentum, the particles tend to travel in a straight line, and as a result, particles located at or near the center of the flow line will strike or impact the fiber and are removed. Generally, larger particles will more readily deviate from the flow lines than smaller ones. In practice, because the differential densities of the particles and liquids are very small, this mechanism is less effective in liquid filtration.
For particles that have little mass, separation can result from diffusional interception. In this mechanism, particles collide with the liquid molecules, which cause the particles to move randomly around the fluid flow lines.
Such movements, observed only on a microscopic level, are called Brownian motion. Motion causes the smaller particles to deviate from the fluid flow lines and increases the likelihood of their striking the fiber surface and being removed. However, as with inertial impaction, this mechanism has only a minor role in liquid filtration.
Equally effective in liquids and gases, direct interception is the predominant mechanism for removing particles from liquids. In the depth of the filter medium (the medium itself, the cake or the filter aid, as stated previously), not only a single fiber or structure but also a rather tortuous path is visible. This tortuous path defines the pores or openings that will remove the solids. If they bridge across the structure or if two or more particles strike a pore simultaneously, particles smaller than the pore diameter might also be removed.
Both inertial impaction and diffusional interception are much less effective with liquids than with gases. Because the density of the particles will be typically closer to that of the liquid than to that of the gas, deviation of a suspended particle from the liquid flow line is much less, so impact on the structure of the medium is less likely. Moreover, impact to the surface of the filter media is not followed by adhesion of the particles to the surface of the filter media. Diffusional interception in liquids occurs only to a very limited extent because Brownian motion is not nearly as pronounced in liquids as it is in gases.
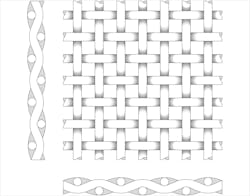
A plain weave wire mesh features a simple over- and under-weave pattern and a straight flow path.
Solid-liquid separation filter aids
In some cases to help the filtration process, filter aids are used to change the solid phase of the material. Filter aids can range from proprietary products such as diatomaceous earth or expanded silica to randomly selected crystalline materials. In general, they are only useful in filtration processes, although in specific cases they can assist settling if the suspension solids tend to adhere to or impinge on the filter aid crystals. Diatomite, perlite and cellulose are the most widely used porous media (filter aids) in dynamic process filtrations, with a high percentage of fine filtration applications using diatomite.
Diatomite is obtained from diatomaceous earth. This sediment is greatly enriched in silica in the form of the siliceous diatoms, which is a diverse array of microscopic, single-cell algae of the class Bacillariophyceae. These diatoms are sufficiently durable to retain their structure through both long periods of geologic time and thermal processing. Diatomite products are characterized by an inherently intricate and highly porous structure composed primarily of silica, along with impurities of alumina, iron oxide and alkaline earth oxides.
Perlite is a naturally occurring volcanic glass that thermally expands upon processing, and it is chemically a sodium potassium aluminum silicate. After milling, a porous, complicated structure is present, but because its structure is not as intricate or tortuous as that of diatomite, perlite is better suited to the separation of coarse micro-particulates from liquids having high solids loading. Perlite is lower in density than diatomite, which enables using less filter media by weight. Both it and diatomite are useful functional filtration components of depth filter sheets and pads.
Considering cellulose & other organic media for solid-liquid separation
Cellulose filter media is produced by the sulfite or sulfate processing of hard woods. Cellulose is characterized by its high aspect ratio, which enables it to easily pre-coat a filter media. It is most often used in combination with diatomite. Like perlite, cellulose possesses a less intricate structure than diatomite.
Other organic media includes potato starch particles, cotton linter, and polymeric fibers and flakes. These materials can help disperse diatomite in some systems or are specific to certain applications. An unusual mineral filter aid of organic origin, the ash from the combustion of rice hulls, has high silica content and a residual carbon char and is useful in waste treatment and stabilization of hazardous materials.
Filter aid can be used as a pre-coat or a body feed. As a pre-coat, the filter aid protects the filter media against the penetration of unwanted solids and premature blinding of the media. In practice a combination of the two approaches is most common. In all cases, remember that this filter aid becomes part of the solids, and under normal conditions no practical way exists to separate them. Thus, they add to the amount of solids that must be disposed.
Selecting the appropriate filter aid depends upon the engineer’s understanding of the application and filtration objective. For example, particle size, settling rate, solids density and, most important, solids characteristics (granular, slimy, coarse, fine or otherwise) are critical parameters to consider. Also consider how the solids to be removed will interact with the filter aid. If the solids form an impermeable layer, then the thickness of the pre-coat does not matter since filtration will stop once the solids layer is formed. If the solids are more fibrous and amorphous and can get into the depth of the pre-coat, then a thicker pre-coat will be beneficial.
Filter media for solid-liquid separation
For the purposes of this article, two main types of filter exist: media synthetic cloth or metal. The choice of media depends on filtration removal efficiency, process requirements, filter technology, characteristics of the solids and liquids, and other parameters such as chemical and thermal resistance.
With synthetic cloths, the materials can be polyester; nylon; polypropylene; PVDF (Kynar); PEEK; or fluoropolymers, such as ETFE, PTFE, E-CTFE, carbonized or polyester. The media can then be segmented by the degree of openness. Plain, square weaves have visible or nearly visible weave openings normally larger than 200 microns and closed weave filter cloth from 1 to 200 microns. For the weave itself, warp is the lengthwise of longitudinal thread in a roll, while weft is the transverse thread.
Media suppliers also talk about monofilament and multifilament. Monofilaments are the most concise and regular fabrics. The singular strand threads are capable of exact detail. They wear well, and solids are less likely to adhere. They are easier to clean and less likely to blind. The surface of the threads is less coarse, or more smooth and polished. Multifilament fabrics are fine strands twisted together into threads. The threads are coarse and have mild elasticity. Multifilaments are harder to clean and can sometimes trap solids. Filter media can also be both mono- and multifilament.
Lastly, different specifications pertain for open area, thread size and pore sizes. Percentages are given that indicate the relationship between the total open area of the mesh and the area that is covered by the threads themselves. A greater percentage of open area permits higher filtration rates. A greater thread size has more strength, but the percentage of open area in the total mesh is diminished. The actual number of threads per square inch of fabric is always the same.
The media suppliers make it even more confusing with different descriptions and nomenclature.
Synthetic cloth weaves include:
• Plain square weave
• Twill weave
• Plain, reverse Dutch
• Double layer weave
Metal media for solid-liquid separation
The alternative is metal media. Metal media can be single layer or multilayer and is fabricated of stainless steel and alloys, such as Hastelloy, Inconel, nickel, Monel and titanium. Single layer metal mesh is similar to the cloth weaves.
Other metal weaves include:
• Plain weave
• Twilled weave
• Plain Dutch weave
• Twilled Dutch
The multi-layered weaves can be sintered or un-sintered. With un-sintered material the interlocking of the weave is the only strength factor that prevents wire movement. Sintering makes the geometry of the original weave permanent, and therefore the pore size is fixed.
Sintered metal media is constructed of multiple layers of wire mesh and designed for precise controlled porosity, uniform pore sizes and distribution. The laminates are permanently bonded under precise diffusion bonding (sintering) conditions. The standard designs can be two, three or five layers. The middle layer excels at removal efficiency while the top layer is protective and the bottom for draining.
The role of coagulants & flocculants in solid-liquid separation
As discussed earlier, filter aids can alter the material phase of the solids. In some situations, to aid in the filtration process, it is necessary to change the effective particle size of the solids with coagulation and flocculation processes. These can only be used if the process itself can tolerate added chemicals. The suspended particles in slurry will vary in size, shape and density. Coagulation and flocculation occur in successive steps intended to overcome the forces stabilizing the suspended particles and to allow particle collision and growth of solids into larger particles to help filtration. If one step is incomplete, the next one will be unsuccessful.
Chemically, coagulant chemicals are metallic salts, such as alum or polymers. Polymers are man-made organic compounds made up of a long chain of smaller molecules. Polymers can be cationic (positively charged), anionic (negatively charged) or nonionic (neutrally charged).
Coagulants are usually fed into the water using one of two methods. A gravimetric feeder moves dry chemicals into the water by weight. A metering pump feeds a liquid into the water by pumping a volume of solution with each stoke or rotation.
The first step destabilizes the particle’s charges. Coagulants with charges opposite those of the suspended solids are added to the slurry to neutralize the negative charges. Once the charge is neutralized, the small suspended particles can stick together. The slightly larger particles formed through this process, called microflocs, are invisible to the naked eye. The water surrounding the newly formed microflocs should be clear. If they are not, all the particles’ charges have not been neutralized, and coagulation has not been carried to completion. In this case, more coagulant may be needed.
Following coagulation, a second process called flocculation occurs. A gentle mixing stage, it increases the particle size from submicroscopic microfloc to visible suspended particles. The microflocs are brought into contact with each other by slow mixing. Collisions of the microfloc particles cause them to bond to produce larger, visible flocs. The floc size continues to build through additional collision and interaction with the polymers added. Once the floc has reached its optimum size and strength, the slurry is pumped to the filtration system. It is important to select the correct pump for the flocked slurry to insure the pump’s shearing forces do not destroy the flocs.
Surface changes
Surface charges on particles can affect the rate and extent of the filtration process. The magnitude of the actual charge is not easily measured, but the effective charge of the solids in the slurry can be characterized by the solid’s zeta potential. What does this mean for filtration? At times, maximum repulsive forces are beneficial to keep the particles discrete and prevent them from going into larger particles and settling. In other cases, the opposite effect is desirable because by removing the forces, the particles are allowed to grow and settle. The real answer depends upon the process conditions and application.
It is important to note that the zeta potential can vary due to the pH and the ionic species strength in the slurry. For example, the zeta potential can be lowered by adding non-adsorbed electrolyte ions to the bulk slurry to promote agglomeration of the solids. Of course these parameters can only be modified if they are compatible with the process.
The intensity of the charge of both the particle and the fiber is critical. Normally, as the charge increases and the particle size decreases, the capture efficiency will increase. Throughout, however, remember that the charge or zeta potential is often unstable and can be influenced by pH, humidity, ionic strength and other factors.
There is much more to say about solid-liquid separation processes. Future articles can address media types in greater detail, as well as relevant issues related to testing, commissioning and operation.
Editor’s Note: This article was adapted from Solid-Liquid Filtration: Practical Guides in Chemical Engineering by Barry A. Perlmutter, published by Elsevier Inc. in 2015.
Barry A. Perlmutter is president and managing director of BHS-Sonthofen Inc., a subsidiary of BHS-Sonthofen GmbH. He has more than 30 years of technical engineering and business marketing experience in the field of solid-liquid separation. He holds degrees in chemistry, environmental science and business administration.
Detlef Steidl is director of application engineering for filtration technology at BHS-Sonthofen GmbH. He has more than 25 years of experience in the chemical industries and has an advanced engineering degree from Frankfurt University of Applied Sciences.
BHS Sonthofen is involved in machine and plant engineering of process technology, including crushing, recycling and filtration operations.