Closing the gap on technology transfer to unlock speed to market

Life science companies have always been under pressure to bring their products to market more quickly, but these pressures intensified during the industry’s race to find cures and treatments for COVID-19. As a result, many companies have been willing to take on more risk and invest in technologies that support digital operations. Those companies able to innovate faster will have a competitive advantage, and those unable to transition quickly will likely be left behind.
If leveraged correctly, software can bring together data, process and people by providing access to more usable data, facilitating greater collaboration and automating workflows. Innovators mastering data management software are unlocking speed to market through better decision making and faster technology transfer. By helping personnel — from the research and development labs to the production floor — make faster, better decisions at every stage of the product development lifecycle, these organizations are gaining competitive advantage through faster product releases.
Supporting better decision making and faster technology transfer means rethinking the way data is handled across the product development lifecycle. It starts with building a culture of standardization using software tools supporting easier technology transfer across the drug development cycle. Putting a strong technology transfer solution in place for the development cycle builds a foundation for the emerging technologies that will extend those same benefits across the gap between development and manufacturing, holistically managing the life sciences lifecycle from idea to delivery.
Today’s tech transfer challenge
In the early years of pharmaceutical manufacturing, records were kept on paper, which could easily be disorganized, lost or inadvertently destroyed. Most life sciences companies today have moved to digital records instead of paper, but in many cases, the management and organization of those digital records still echoes the paper trails of the past.
Even when documentation is created electronically, such as in spreadsheets, the data is often scattered across many different systems. Scientists in research and development start developing their products individually or in small groups, with notes, records and results all spread out across multiple locations within the organization.
In the most complex environments, different departments may even use different proprietary software packages, making it even more difficult to share data. When activities are performed, the changes — and the important reasoning behind them — are all recorded in this same siloed set of documents.
In addition, when manual activities and data collection and storage are performed globally, and over many years, it is difficult to go back through records and quickly see where changes were made when there is a problem. To stay competitive, life sciences organizations are exploring ways to make critical data more accessible. As a treatment passes from one stage of development to the next, tech transfer teams must find ways to move the accompanying data and make it accessible to the next groups in the development lifecycle.
Building a culture of standardization in development
To speed technology transfer, organizations are adopting best practices using standardized information, contained in the same fit-for-purpose knowledge management software, through the entire development process.
Process knowledge management (PKM) software captures every decision made in the product development process electronically. Data is not only more reliable, but it is also standardized in format and access method, making it more intuitive to locate information, share it and make it easy to comprehend. Internal calculation engines manage process parameters and calculations without the need for external spreadsheets by instead storing data in a database accessible across development.
The most advanced PKM software packages promote standardization via templates, so teams can use common definitions and keep them up to date over time. For example, using these templates, development teams can push changes to multiple recipes at one time, saving many hours of manual editing. Moreover, the PKM software tracks the changes, providing a clear audit trail and displaying how the change impacts each recipe.
Moving a product across the many stages of tech transfer to quickly bring it to market requires a great deal of collaboration. Using cloud-based storage, PKM software makes all contextualized data available to all team members, without the need to train each of them on how to navigate a variety of different systems (Figure 1). They can easily and securely access recipes, attach documents and utilize built-in workflows — all from the same tool — from anywhere across the globe.
Groups across the product development process are also leveraging new strategies to simplify compliance. The tools in PKM software use intuitive user interfaces to standardize the product and process specifications that conform to ISA-88 standards. Automatic compliance tools eliminate manual data collection and review cycles. Sensitive, proprietary information — which will become even more important with the move toward personalized medicine — is stored in one location, making it far less likely to get lost, accidentally mixed up with other data or moved offsite through insecure hardware, such as removable drives.
Extending standardization across the gap
While there are many potential opportunities for complications during the development process, the most difficult tech transfer is typically from development to production. Today, process development is typically fully siloed from manufacturing.
Commonly, development and production each perform steps their own way using proprietary tools. Trying to move from one area to the next means accounting for all the previously stored data, and then transferring this data from bench-scale to production-scale equipment. At best, this process typically takes months, and often takes years when performed manually.
Implementing PKM software builds a bridge between development and manufacturing. The most advanced PKM tools are designed to serve the needs of both development and manufacturing teams, while maintaining standardization of data, interface and usability. By selecting a fit-for-purpose PKM solution, organizations can improve tech transfer not just across development, but across the entire drug development and production lifecycle.
Today, knowledge management tools are being adapted to not only work in the development cycle, but to support the tools used in manufacturing, making it easier to complete the additional tech transfer process between development and production. When development uses a PKM tool designed to seamlessly integrate with manufacturing tools, master recipes are more easily transferred.
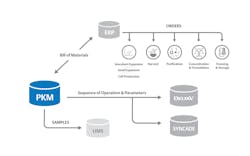
With these types of integrated systems, the recipe repository is directly linked to automation software, such as the distributed control system and the manufacturing execution system. In a few short steps, the PKM system seamlessly pushes sequence of operation and parameters down to these systems, ensuring downstream teams know exactly what to purchase, and how to manufacture drugs based on the pre-established link from recipes to the execution system (Figure 2). Under the right circumstances, this automated process can help reduce the time to market from 10 years to 2.5 years.
The benefits also extend to products already on the market. If a new treatment has a problem after release, knowledge management across the lifecycle provides easy access to the data needed to quickly solve that problem. This is particularly important because when something goes wrong, everything is put on hold until the organization can provide a solution and its associated support data.
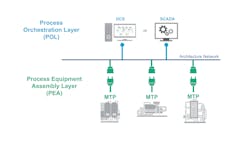
Another way organizations are speeding technology transfer in manufacturing is with flexible manufacturing strategies. Life sciences disruptors are embracing technologies, such as module type package (MTP), to enable near plug-and-play connectivity among various items of equipment, such as process skids. With MTP, organizations do not spend weeks or months trying to integrate equipment when moving from bench- to production-scale. Teams can easily add new equipment without having to worry about compatibility, and they can often more easily use existing equipment on-site to run processes faster (Figure 3).
Conclusion
Life sciences manufacturing has changed. Patients and practitioners now expect faster, more specialized treatments — a shift that will resonate across the industry and change the way organizations operate, from the ground up. The life sciences organizations poised to win the future of manufacturing are already considering ways to better standardize and integrate processes for faster tech transfer. At the heart of these strategies are software and technologies like PKM and MTP, which build a foundation to enable faster movement within and between development and manufacturing to meet these changing needs.
Kristel Biehler is vice president of life sciences for Emerson’s process systems and solutions business where she leads the day-to-day business activities in sales, operations and technology that serve the life science industries. In her previous role at Emerson, she was the automation solutions vice president of sales for the western United States where she led teams that helped customers identify, architect and implement automation and digital strategies across a wide range of industries. Biehler started her career with Emerson in 1998. She holds a bachelor’s degree in mechanical engineering from the University of Utah. Prior to Emerson, she worked for Sorex Medical as an automation engineer.
Emerson
About the Author
Kristel Biehler
Vice president of life sciences for Emerson’s process systems and solutions business
Kristel Biehler is vice president of life sciences for Emerson’s process systems and solutions business where she leads the day-to-day business activities in sales, operations, and technology that serve the life science industries. In her previous role at Emerson of five years, she was the automation solutions vice president of sales for the western United States where she led teams that helped customers identify, architect, and implement automation and digital strategies across a wide range of industries. Kristel Biehler started her career with Emerson in 1998. She holds a bachelor’s degree in mechanical engineering from the University of Utah. Prior to Emerson, she worked for Sorex Medical as an automation engineer.