Closed-system dispensing is better for bulk-chemical applications
Liquid chemicals — costly and sometimes dangerous — are often packaged and transported in drums, jerry cans and IBCs for delivery to distributors and users. The common open-system method of dispensing chemicals from bulk containers is fraught with complications and can present dangers to people, processes and the environment.
Figure 1. Benefits by application; A closed dispensing system, by virtue of its design, offers more value as safety concerns and cost per container increase. The chart illustrates the potential contribution of closed system dispensing to applications in each of the industries profiled in this paper.
Spills caused by operators or malfunctioning equipment create slip-and-fall hazards and risk exposing workers to contact with hazardous chemicals. Fumes that escape poorly sealed bulk-chemical containers during dispensing can irritate or sicken workers. Chemicals themselves perhaps must be kept from contamination or oxidation during dispensing, particularly when applications require purity, or if the media is highly valuable or spoils from air contact.
A veritable alphabet soup of regulating bodies — including the EPA, DOT, UN, FDA, NSF, OSHA and others — impact how chemicals are transported, stored and dispensed. Industry standards may require use of animal-free materials or sustainable practices.
There are hundreds or even thousands of different bulk chemical dispensing applications, and each brings its own set of considerations when it comes to the rationale for closed-system dispensing (See Figure 1).
One way to mitigate safety and regulatory concerns and costs is to implement a closed, or sealed, system for dispensing hazardous or costly chemicals.
Benefits
To start, a closed-dispensing system addresses and mitigates chemical exposure and contamination concerns during transport, dispensing and disposal.
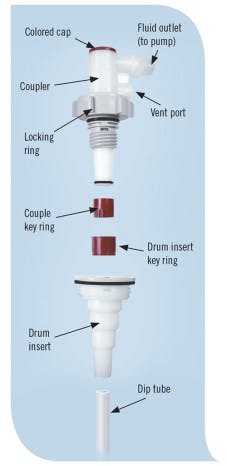
Figure 2: A closed dispensing system makes use of a bung plug with dip-tube assembly to extract liquids from rigid containers to minimize spills, fumes and environmental impact.
Closed systems usually have a reusable coupler/dispense head that connects to an integrated bung plug with dip-tube assembly (See Figure 2). The coupler should have a ported-vent system for vapor management that allows make-up air or a nitrogen blanket gas into the drum or tote to replace the liquid being removed, as well as an automatic shutoff valve that stops flow upon disconnection. The dip-tube system seals and travels with the container to the end user site. Once there, the coupler connects to the dip-tube assembly for dispensing. This makes it an ideal system for meeting some of the general dispensing goals in many applications.
A closed dispensing system addresses unique application concerns, as well.
For example, in high-purity applications where contamination is a consideration, a closed system featuring cleanroom-manufactured components can ensure contaminants are not introduced in system manufacture.
Some dispensing-equipment manufacturers also produce closed-system components that meet FDA and animal-free specifications, thereby reducing even further the risk of contaminants prohibited by industry or government regulations in applications where end products will be used or consumed by humans.
Many other applications have unusual dispensing container styles, such as those with so-called Nalgene bottle caps. In these applications, it’s best if a universal adaptor option is available. The DrumQuik system from CPC, for instance, offers a three-port universal dispensing adaptor (Figure 3). The adaptor features ¾" male NPT threads that mate with common containers’ bung, plugs and caps, allowing simultaneous chemical extraction, venting/application of a blanket gas and recirculation — if needed.
Regulatory details
OSHA addresses the management of highly hazardous chemicals (HHCs) in its guidelines for Process Safety Management (PSM). A performance-oriented standard, PSM protects workers and the workplace when a threshold quantity (TQ) of highly hazardous chemicals exists within an area under an employer’s control. Chemical dispensing — of industrial organics and inorganics as well as pharmaceuticals — is an activity that falls within this definition. For example, the chlorine used in potable water-dosing applications has a TQ of 1500 pounds, making this application subject to PSM requirements.
Figure 3: For special applications, a three-port adaptor allows simultaneous chemical extraction, venting/application of a blanket gas and recirculation.
A wide range of industries deals with the transport and mixing of hazardous and/or valuable chemicals, and closed systems dispensing can offer safety and other benefits for chemical handling, processing and shipping applications. Many manufacturers are routinely audited by the EPA, FDA and local governments to ensure their work environments are free of harmful pollutants. Integrating a bung plug with dip-tube assembly and a sealed coupler/dispense head can help establish a level of security within an operation, maintain a safe work environment for employees, and help provide superior contaminant-free products for customers.
Thomas A. Braun is a business manager, chemical and packaging products, Colder Products Co. (CPC).
CPC provides quick-disconnect couplings and fittings for life sciences, industrial and chemical-handling markets. Its range of chemically resistant, quick-disconnect couplings and DrumQuik® closed chemical dispensing systems provide non-spill operation and improve employee and environmental safety. Applications include water treatment, janitorial and sanitation, Diesel Exhaust Fluid (DEF) dispensing, semiconductor and pharmaceutical manufacturing as well as pump, filter and de-ionized water connections.