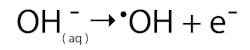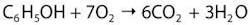The modern world depends on the use of stable organic water soluble compounds for many processes, including chemical processing, adhesives, pesticides, dyes, preservatives and pharmaceuticals. Often based on phenols, a hydroxyl group bonded to an aromatic hydrocarbon group, these can cause a problem in wastewater management because they are resistant to breakdown by oxidation and are frequently toxic to the organisms in biological water treatment plants.
Figure 1. Above. A schematic of a microwave plasma enhanced chemical vapor deposition reactor used to manufacture free-standing Electrochemical Processing Grade CVD diamond electrodes.
The hydroxyl radical is a strong oxidant, second only to fluorine in power, which can be used to treat these dissolved recalcitrant pollutants in processes that are collectively known as advanced oxidation processes (AOPs). These water treatment techniques can eliminate almost all types of toxic and hazardous dissolved organic compounds in the aqueous phase via oxidation.
For many applications wet air oxidation processes are very effective. These operate at high temperatures between 150°C to 350°C and pressures of 10 to 220 bar. In smaller scale applications where operation at low pressures and low temperatures of 20°C to 60°C are desirable, many of the low temperature AOPs are being held back from widespread adoption due to their relative complexity and high treatment costs.
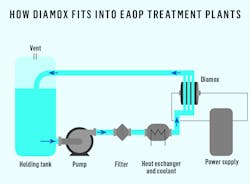
A recent development in advanced oxidation technology that utilizes unique freestanding boron doped diamond electrodes addresses these concerns. Diamox is a electrochemical cell that generates the hydroxyl radical electrochemically. The packaged reactor is simple to implement into on-site industrial wastewater treatment systems, providing an environmentally cleaner and versatile solution that can be used across various effluents. Its latest design has been successfully applied in a pilot project with an industry-leading wastewater treatment company, delivering unparalleled electrochemical oxidation capacity that can be scaled to meet industry requirements.
At ~600 µm thickness, these 138 mm diameter electrodes freed from a substrate have an application life of many years.
Hydroxyl radical generation
Traditionally, generating and utilizing the freest of free radicals in water treatment presents challenges for the water treatment processes. The conventional low temperature approach to generating the hydroxyl radical directly in the effluent stream has been to mimic the upper atmosphere chemistry that naturally cleans the air. This method adds strong oxidizing species, such as ozone, hydrogen peroxide or oxygen, to the effluent and via additional energy input, such as ultraviolet (UV) radiation, to trigger hydroxyl radical generation.
These processes often require complex sequencing of dosing, UV light exposure and multiple oxidizing reagents to be effective, and they can be difficult to manage in a water treatment environment. For example, handling one of the constituents of rocket fuel presents safety and logistical challenges and is expensive. As a result, implementing these types of advanced oxidation water treatment processes has been constrained to low level contaminated waste streams, and uptake has been fairly restricted by the shear complexity and cost.
Figure 2. A schematic of a bipolar electrochemical cell
Boron doped diamond electrodes
Diamox is the result of more than 20 years of research into electrochemical oxidation, leveraging boron doped diamond (BDD) and expertise in chemical vapor deposition synthesis and engineering synthetic bulk-free standing polycrystalline chemical vapor deposited diamond. When heavily doped with boron, diamond — a wide band gap semiconductor — can be engineered to have metal-like conductivity while retaining all of its physical properties, making it the most chemically inert and robust electrode material available. With no substrate to degrade, the technology contains polycrystalline BDD electrodes that have a field lifetime measurable in years in electrochemical treatment conditions that no other electrode materials can withstand for even a few hours.
Electrochemical advanced oxidation
Diamond is renowned as a stable allotrope of carbon. In nature it survives for billions of years, and this chemical inertness is significant in the electrolysis cell. Instead of electrolyzing water (H2O) to evolve oxygen gas (O2), a weaker oxidant, it has the potential to oxide the hydroxyl ions (OH-) that naturally occur in water to generate the hydroxyl radical (OH). At the anode, the reaction occurring is simply expressed as:These are very short-lived species appearing at the anode surface, and boron doped diamond’s resistance to oxidation by hydroxyl radicals enables them to be temporarily captured (adsorbed) onto the surface available to oxidize dissolved pollutants in wastewater streams. Hydroxyl radicals have the oxidation potential to fully oxidize, or mineralize, species such as phenol, a recalcitrant water soluble biocidal water pollutant.
At the anode surface, the hydroxyl ions are oxidized to form the radical that is able to oxidize the phenol molecule. In total it requires 28 electrons to be transferred into the anode surface for the phenol molecule to be mineralized into carbon dioxide gas. The process is entirely driven by the current flowing through the electrochemical cell and is controlled and the rate of mineralization limited by it. However, because the diamond electrode surface isFigure 3. A water treatment system equipped with Diamox requires these service requirements: electrical power, cooling to keep process temperatures below 50°C and vented extraction of the effluent tank.
When efficiency is defined as the ratio of desired species oxidation to undesired oxidation of water molecules at dissolved organic contamination concentration of greater than 10,000 mg/l, Diamox electrochemical cells are capable of operating at around 100 percent of current efficiency. The voltage required to drive this process is governed by the oxidation potential needed to drive the generation of hydroxyl radicals and the conductivity of the effluent.
Diamox electrochemical cell
Diamox is a specially designed electrochemical cell that packages freestanding boron doped diamond electrodes that are manufactured by microwave chemical vapor deposition process. A bipolar electrochemical cell has an electrode stack comprising of 21 electrodes in a 20-cell system. Here the power is fed in from each end electrode, while the two surfaces of the inner electrodes act as cathodes and anodes sequentially.
This latest technology has been significantly redesigned with increased capacity and improved efficiency for more cost-effective treatment of waste streams. Through a combination of increasing the electrode area and the power density that the cell operates at, the new 20-cell version has five times the oxidation capacity of earlier generations. Capable of operating at current densities of 30,000 Amp/m2 from 45 to 250 kilowatts (kW), the new design has the power and capacity to mineralize dissolved pollutants at up to 2 kilograms (kg) of chemical oxygen demand (COD) per hour.
The electrochemical advanced oxidation process has a number of advantages. The treatment process is driven solely by the electrochemistry. No additional strongly oxidizing reagents need to be added to the effluent. Additionally, it does not depend on a combination of photocatalytic reactions for the formation of the hydroxyl radicals. The universally effective non-discriminatory process can treat a range of dissolved contaminates, and it delivers a compact process and does not require large secondary treatment process.
Figure 4. A typical COD reduction curve for a recalcitrant phenol containing effluent stream. With a COD concentration of around 10 percent at the start of the process, this highly conductive effluent stream can be treated efficiently using Diamox.
In the context of conventional water treatment technology, the new version is suited for small to medium volumes of difficult-to-treat effluent streams that are otherwise transported to specialized facilities. The high current capability gives it capacity to treat relatively high concentration effluents, COD >10,000 mg/l, the modular nature enables it to be readily tailored to match application from 0.2 to 10 m3 per hour.
Water treatment technology integration
Diamox’s small footprint packaged product can be scaled in size for incorporation into any existing or new industrial water treatment plant, which enables on-site treatment systems that are simple to operate and maintain. It also has the versatility to work with most effluents — an advantage over other advanced oxidation processes that only treat specific types of waste.
Diamox was recently leveraged in a successful pilot project by an industry-leading wastewater treatment company for use in treating highly contaminated, spent caustic streams, such as those generated by refineries producing clean fuel. In a series of treatment studies, the product was proven for different mixed spent caustics from a refinery process to reduce the COD by more than 90 percent with an output that is safe to discharge into the environment.
Conclusion
Diamox is a new generation of electrochemical advanced oxidation cell technology. When integrated into an electrochemical wastewater processing unit, it is effective in treating contaminated industrial wastewater that cannot be treated by biological methods. This packaged reactor is simple to implement into on-site industrial wastewater treatment systems, providing an environmentally cleaner and versatile solution that can be used across various types of effluents with no hazardous chemical additions.
Dr. Tim Mollart leads the research and application of Element Six’s synthetic diamond electrode systems. Mollart works closely with customers and academic institutes in the industrial wastewater space, supporting them with next-generation synthetic diamond solutions for extremely toxic water. He is based in the U.K.