Extractive distillation: What is it and when should it be used?
Extractive distillation is a powerful technique utilized to separate close-boiling and azeotropic mixtures. As it gains wider consideration within industrial chemical processes, it is important to understand the inner workings and possible applications. Discussed here is an introduction to this technology and its corresponding mathematics, as well as examples that illustrate the use of extractive distillation in real-world applications.
A brief refresher on distillation
Separations processes are an integral part of industrial chemical processing for the recovery and purification of products and solvents. Among the many different separation methods available, distillation is the most ubiquitous. In fact, most production of commodity chemicals includes distillation.
During distillation, the components of a liquid mixture are separated by exploiting differences in boiling points. Boiling point measures how readily a component vaporizes, specifically, its volatility. Volatile liquids have low boiling points, while less volatile liquids have high boiling points. However, the energy required to vaporize a molecule of any liquid is fairly constant regardless of boiling point.
There are many different types of distillation processes, which vary by either the steps involved or the conditions under which the separation is performed. They include:
- Batch distillation
- Continuous distillation
- Steam stripping
- High vacuum distillation
- Reactive distillation
- Extractive distillation
- Azeotropic distillation
- Pressure swing distillation
The final three aforementioned methods are especially useful for “breaking” azeotropes. These mixtures are comprised of components that have constant boiling temperatures at which the liquid and the vapor compositions are equal at a given pressure, posing separation challenges not addressable by conventional distillation methods.1
Introducing extractive distillation
Extractive distillation works by introducing a solvent to modify the molecular interactions of a mixture. The solvent alters relative volatilities by changing the intermolecular interactions of the components within the mixture. This ultimately allows one of the other components to be driven overhead as a distillate product with high purity.2 Typically, the solvent added in extractive distillation has a higher boiling point than either of the feed components, and thus, is easier to recover for reuse. In azeotropic distillation, the solvent forms a new azeotrope with one of the components. Given the similarity with azeotropic distillation, extractive distillation was previously considered to be a special case of azeotropic distillation in a double-feed column, deemed suitable for the separation of close-boiling mixtures by using a solvent that would not form any new azeotrope.1 However, the two processes are now considered distinct since they obey different feasibility rules and operate using different column configurations. In addition, extractive distillation is easier to model via process simulations due to the absence of two liquid phases, normally present in azeotropic distillation. In this way, extractive distillation is often regarded as a preferable and easier method than azeotropic distillation.
There are other distillation processes used to break azeotropes that do not involve the introduction of solvent into the process, such as pressure swing distillation, which achieves separation by taking advantage of a shift in composition of an azeotropic mixture with pressure.2 Nonetheless, there are many reasons why pressure swing distillation may not be the ideal process for “breaking” azeotropes. For example, some systems do not exhibit a significant variation in azeotropic composition over the pressure range. This can become a cost-prohibitive solution due to the high energy usage required for a minimum-boiling azeotrope or the need for larger diameter columns to accommodate high flow rates. Also, operating at elevated pressure, which also increases the operating temperature, can result in thermal instability in some cases.
The process
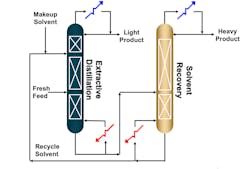
Extractive distillation involves at least two columns, depending on the complexity of the solvent recovery process. In the first column, there are two separate feeds. The “fresh feed,” which contains the solution to be distilled, is added in the lower part of the column, and the solvent feed is added higher up in the column (Figure 2).
These feeds combine and are then processed through the three sections of the extractive distillation column: solvent recovery, rectifying and stripping (from top down). Solvent recovery refers to the trays between the solvent feed tray and the top of the column. This is where the component of higher relative volatility is concentrated and leaves as a distillate product. Reflux is required to separate it from the solvent and provide “reflux” to the rectifying section. Moving down the column, the rectifying section includes the trays between solvent feed and fresh mixture feed. In this section, the component of lower relative volatility is removed from the light component. The liquid and vapor composition at the top of this section is relatively free of the heavy component. Finally, the stripping section refers to the trays between the mixture feed tray and the bottom of the column. This is where the heavy component is concentrated and removed as the bottom’s product. Both the heavy component and solvent are subsequently introduced into a recovery column in which the solvent is removed as the bottom’s product and recycled back into the first column, and the distillate is relatively pure heavy component.
Solvent selection
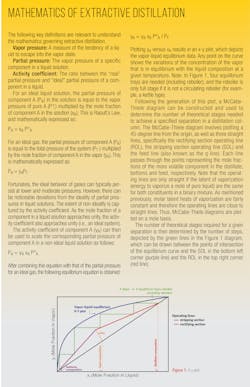
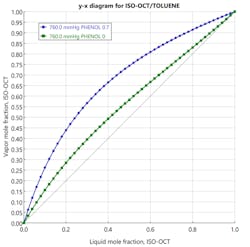
When choosing a solvent for an extractive distillation process, its boiling temperature should ideally be higher than the “fresh feed” components. It should also be miscible with the mixture without forming an azeotrope. Additionally, it should alter the activity coefficients of the components, thereby causing their relative volatilities to change. The performance of potential solvents can be predicted by generating a McCabe-Thiele diagram (Figure 3) of the binary mixture in the presence of a fixed concentration of a chosen solvent plotted on a solvent-free basis. If the diagram shows that the binary separation can be enhanced by the addition of a solvent, the next step is conducting a series of process simulation runs, subsequently supported by empirical pilot-plant studies. Figure 3 shows that adding phenol as a solvent to an isooctane/toluene solution should yield positive results through an extractive distillation process.
When solvents are ranked in order of increasing relative volatility (or selectivity), the solvent ranked highest is typically considered to be the most promising solvent for a given separation task. From an economic viewpoint, this solvent will also always give the lowest total annual cost of the extractive distillation process.4
System examples
Component mixtures with low relative volatility or those containing azeotropes are good candidates for extractive distillation. Using the example stated in the plot above, consider a system consisting of toluene (boiling point 110.8˚C) and isooctane (boiling point 99.3˚C). Although the separation is difficult using a conventional distillation process, extractive distillation proves to be successful with the addition of phenol (boiling point 181.4˚C) as solvent. In practice, toluene and isooctane separately form non-ideal liquid solutions with phenol. Consequently, when all three substances are combined, the toluene and isooctane themselves behave as a non-ideal mixture, and their relative volatility becomes high.7 The isooctane is recovered as a distillate product, while the toluene-phenol mixture is recovered as the bottom’s product. This mixture is further separated in a solvent recovery column wherein toluene is distilled away from phenol. Finally, phenol is recycled back to the first column.
Another example to consider is an azeotropic system consisting of water and tetrahydrofuran (THF). DMSO has been shown to be an effective solvent to achieve separation through extractive distillation. It strongly associates with water through hydrogen bonds, while negatively combining with THF, a proton receptor. In fact, the water/THF azeotrope disappears when the system contains just 10% DMSO.8 Although the separation of this mixture can also be achieved using pressure swing distillation, an economic evaluation carried out using Aspen Process Economic Analyzer indicated that, for the same scale of production and the same product purity, the utility cost of pressure swing distillation was 5.6% higher than that of extractive distillation, and the total annual cost (including the cost of solvent makeup) of extractive distillation was 1.69% lower than that of pressure swing distillation.9
Applications of extractive distillation
Extractive distillation has proven to be useful in many different fields, including but not limited to the pharmaceutical, chemical, petrochemical and refining industries.3,4 One of the major applications of extractive distillation in the petrochemical and refining industry is the recovery of aromatic hydrocarbons. Advanced solvent technology has increased the feasibility and profitability of using extractive distillation, resulting in benefits such as lower capital cost, lowered risk of product contamination with solvent carryover, and high product recovery and purity.3 As a result, this industry currently uses extractive distillation processes for the recovery of pygas byproducts such as benzene, toluene and xylene (BTX) from naphtha-based steam crackers, and in the desulfurization of FCC gasoline and styrene recovery from pyrolysis gasoline.3,5
In the pharmaceutical and specialty chemical industries, extractive distillation may further play a role in solvent wastewater treatment. For example, isopropyl alcohol (IPA), one of the most widely used solvents in the manufacturing of pharmaceuticals, can be obtained from an IPA/water mixture as the top product of an extractive distillation column after the addition of ethylene glycol.6 The recovery of solvents such as IPA have many benefits, including waste reduction, cost savings and meeting stringent EPA requirements for wastewater disposal.
Extractive distillation is a powerful tool to separate close-boiling mixtures or azeotropic mixtures. It can make it easier to keep the solvent in liquid phase and require less processing versus azeotropic distillation where the solvent must be condensed before being returned to the initial column. Additionally, extractive distillation can be much more cost effective than pressure swing distillation. Ultimately, extractive distillation is not a panacea for all difficult distillation separations, but it should always be evaluated as an option.
References
- Gerbaud, V.; Rodriguez-Donis, I.; Hegely, L.; Lang, P.; Denes, F.; You, X. Review of Extractive Distillation. Process Design, Operation, Optimization and Control. Chem. Eng. Res. Des. 2019, 141, 229–271. https://doi.org/10.1016/j.cherd.2018.09.020.
- Wang, Y.; Zhang, Z.; Zhao, Y.; Liang, S.; Bu, G. Control of Extractive Distillation and Partially Heat-Integrated Pressure-Swing Distillation for Separating Azeotropic Mixture of Ethanol and Tetrahydrofuran. Ind. Eng. Chem. Res. 2015, 54 (34), 8533–8545. https://doi.org/10.1021/acs.iecr.5b01642.
- Gentry, J. C.; Kumar, Sam.; Wright-Wytcherley, R. Use Extractive Distillation to Simplify Petrochemical Processes; Advances in Solvent Technology Enable Using Extraction Methods to Separate Close Boiling Point Compounds Cost-Effectively. Hydrocarbon Processing. June 2004, p 62+.
- Lei, Z.; Li, C.; Chen, B. Extractive Distillation: A Review. Sep. Purif. Rev. 2003, 32 (2), 121–213. https://doi.org/10.1081/SPM-120026627.
- Pygas upgrading for European steam crackers https://www.digitalrefining.com/article/1000578/pygas-upgrading-for-european-steam-crackers#.YMOscPlKiM8 (accessed 2021 -06 -11).
- Van baelen, G.; Vreysen, S.; Gerbaud, V.; Rodriguez, I.; Geens, J.; Janssens, B. Isopropyl Alcohol Recovery by Heteroazeotropic Batch Distillation. 6th European Meeting on Chemical Industry and Environment 2010.
- Richardson, J. F.; Harker, J. H.; Backhurst, J. R. CHAPTER 11 - Distillation. In Chemical Engineering (Fifth Edition); Richardson, J. F., Harker, J. H., Backhurst, J. R., Eds.; Chemical Engineering Series; Butterworth-Heinemann: Oxford, 2002; pp 542–655. https://doi.org/10.1016/B978-0-08-049064-9.50022-9.
- Zhang, Z.; Huang, D.; Lv, M.; Jia, P.; Sun, D.; Li, W. Entrainer Selection for Separating Tetrahydrofuran/Water Azeotropic Mixture by Extractive Distillation. Sep. Purif. Technol. 2014, 122, 73–77. https://doi.org/10.1016/j.seppur.2013.10.051.
- Liu, X.-Y.; Shang, D.; Liu, Z.-Y. Comparison of Extractive and Pressure-Swing Distillation for Separation of Tetrahydrofuran-Water Mixture. 2017. https://doi.org/10.3303/CET1761235.
Alan Erickson is vice president at Koch Modular and a member of the executive team. Erickson has more than 40 years of experience in process design, startup and project management of chemical manufacturing plants. His subject matter expertise includes computer simulations of entire recovery plants, design of unit operations such as evaporation, distillation, liquid-liquid extraction, absorption and adsorption, heat transfer and fluid flow, complete process control systems and instrumentation. He is a Professional Engineer and holds a B.S. in chemical engineering from Rutgers University.
Koch Modular
About the Author
Alan Erickson
Vice president at Koch Modular
Alan Erickson is vice president at Koch Modular and a member of the executive team. Erickson brings over 40 years’ experience in process design, startup and project management of chemical manufacturing plants. Subject-matter expertise includes computer simulations of entire recovery plants, design of unit operations such as evaporation, distillation, liquid-liquid extraction, absorption and adsorption, heat transfer and fluid flow, complete process control systems and instrumentation. He is a Professional Engineer and holds a B.S. in chemical engineering from Rutgers University.