Process manufacturing’s future is built on remote solutions
Life sciences manufacturing has changed. Most obviously, a global pandemic dramatically altered the way plants operate. Management was forced to stagger shifts to enable social distancing, often stranding key personnel outside the plant when their services were needed. But while COVID-19 made this personnel shift more visible, the reality is the change has been coming for many years. Driven by staffing shortages caused by retirements of experienced workers and the challenge of hiring new personnel, organizations simply cannot retain all the experts they need.
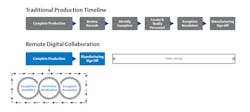
As shift rosters thinned out, plants turned to one of the best solutions available: remote monitoring. Nearly overnight, plant management began implementing methods to empower operators, engineers and technicians to closely monitor production and equipment from wherever they were — with varying levels of success. But even if global pandemics and a wave of retirements disappeared tomorrow, the trend toward remote monitoring is here to stay as a critical enabler of life sciences manufacturing strategies of the future. Building a strong digital manufacturing foundation is key to enabling emerging remote technologies that will empower personnel, improve production and performance, help eliminate contamination, and ultimately unlock lights out manufacturing (Figure 1).
Empowering personnel with remote monitoring
Digital manufacturing has been critical to the remote work that helped life sciences organizations navigate the pandemic and in helping support the more mobile, remote workforce necessary to meet the changes still ahead. The organizations that struggled most with the transition to remote monitoring were the ones still using paper systems. These teams quickly discovered the need to be onsite for many of the tasks well-suited for remote work, such as sign-offs for quality assurance and exception management. Paper-based teams also faced more difficulty monitoring production to maintain peak unit performance.
Conversely, teams in the process of transitioning from paper to digital systems discovered they already had the beginnings of a platform for remote connectivity. Having essential plant data in a digital format enables the use of digital automation tools designed specifically for connecting to real-time process data from the control system, historians, manufacturing execution systems (MES) and more.
Organizations building a remote framework leverage their digital data through secure mobile tools to ensure key personnel receive real-time, intuitive alarm notifications on their mobile devices. Persona-based alerts provide constant awareness of plant and process health, even when users cannot be on site. Leveraging data provided by the control system and asset monitoring software, these alerts provide context and trends to help users inside or outside the plant monitor automation assets and production processes remotely.
Using the information available in digital systems, remote personnel can more easily determine when they need to be on site to solve a problem or if they can assist on-site personnel remotely. Moreover, they perform both local and remote support armed with knowledge of the problem, helping improve efficiency and effectiveness when they arrive.
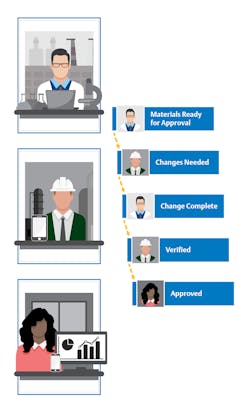
The digital foundation supporting basic remote monitoring can also be expanded, empowering both manufacturing and quality assurance teams to monitor exceptions and determine whether those exceptions have an impact on the final product. Then, using digital tools such as electronic batch record systems, the same personnel can review and sign off from wherever they are, reducing the risk that quality assurance steps will delay product release (Figure 2).
Moving forward, many of these same organizations are implementing higher level applications — such as predictive maintenance, analytics and planning and scheduling software — in a private cloud, unlocking better visibility through remote access any time, from anywhere.
Improving plant production and performance
Though COVID-19 made it difficult to start or continue capital projects, the need for process modifications or expansions to improve production and performance remained. In fact, when facilities were at their lowest staffing in history, many organizations were in the middle of projects they needed to see to completion. The need for new treatments and vaccines did not go away, so these organizations leveraged remote solutions to continue innovating.
Simulation and the cloud
Simulation and digital twin technologies improve opportunities for the development and testing of new control strategies, and then provide a path to successfully implement those strategies even when site staff are limited. Digital twin simulations create replicas of the manufacturing environment, allowing testing of new equipment and strategies without the need to access live equipment. Hosting these simulations in the cloud further empowers personnel to contribute, allowing process developers to test new control strategies from on the plant floor, in the company headquarters, from home or even from another plant across the globe.
With global access to engineering and testing tools, new capital projects are no longer held up while waiting for key personnel and equipment to travel between sites. Project teams can plug systems into a simulation offsite to make control technologies think they are talking to real equipment for comprehensive virtual testing. Much of the project work can be done remotely before equipment even arrives, limiting the time and the number of people needed on site for installation and operational qualification.
Enabling experts around the globe to access high-fidelity simulations makes it easy for life sciences organizations to quickly add new production lines. Teams can perform all the prep work for the new product line in parallel via cloud simulations and remotely support qualifications. When they move on site, the teams simply move the configuration from the cloud to the local automation system and rapidly shift to production. These project strategies are particularly important as manufacturers support rapid production of vaccines without bringing more people into the plant. After projects have been implemented, the simulations retain their usefulness, helping personnel train on the new strategies.
The same strategies can also be applied to factory acceptance tests at OEMs and equipment providers. Using remote and virtual factory acceptance testing tools, project teams can configure and test equipment on simulations — making it easy for key stakeholders to participate from anywhere. When stakeholders are sure the configurations are correct, the team can transfer them to actual equipment for final testing, all at the equipment provider’s site.
Edge computing and control
One of the key toolsets unlocking cloud connectivity for more remote work is edge computing and control. New edge devices designed for secure, one-way communication easily move data between the manufacturing edge and the cloud to enable access by any authorized person from anywhere. Data is collected, contextualized and made accessible from shared data applications — such as a manufacturing repository — for remote access by experts and analytics reporting tools.
Because edge connectivity makes it easy to bring rich data from the plant floor to the enterprise, organizations can get more done with a smaller number of people. Instead of having experts at every plant, an organization can focus on highly experienced mobile experts who can support a wide range of sites without the need to travel to each one. And that same easy movement of data will also allow for cross-site comparisons as organizations build out identical sites in different locations to mitigate supply chain and treatment delivery interruptions.
Eliminate contamination and unlock lights out manufacturing
As life sciences organizations embrace more tools to quickly deliver highly contextualized data around the globe, they will unlock new potential for remote maintenance and operation. Augmented reality (AR) and virtual reality tools will empower personnel, helping local workers take advantage of expert knowledge and gain remote access to live experts.
Using advanced AR technologies in tandem with contextualized data from the control system, experts in a remote location will see exactly what a less experienced person sees in the lab or on the plant floor. Taking advantage of the first-person viewpoint from the technician on the floor, the remote expert will use on-screen tools to annotate and assist, helping guide and upskill personnel around the globe.
Moreover, while today’s remote solutions for process manufacturing are focused heavily on keeping production running even when key personnel are away from the plant, many life sciences organizations are looking at ways to distance physical manufacturing processes from as much human interaction as possible to eliminate opportunities for contamination. The goal is movement toward lights out manufacturing — production and packaging lines that operate entirely closed off from human intervention.
As more areas of production incorporate robotics to create fully clean manufacturing environments, the foundation of remote technology will become even more essential to avoiding production interruptions. Inserting human contact into these clean environments for inspection, adjustments or maintenance has the potential to create lengthy stoppages, both for the work itself and for cleaning the facility to ensure no contaminants are left behind. These stoppages will make it very hard to hit the increased production benchmarks continuous autonomous manufacturing will unlock.
In many cases, technologies like digital twin simulation will help personnel see problems before they even occur in the process, allowing supervisory personnel to make adjustments to avoid issues without intervention in clean sites. Supervisors will have real-time access to alarms in context with relevant data to better understand and diagnose abnormal situations.
As these autonomous operations advance, simulation will be key to success. The most advanced digital twins can constantly update using live process data and run at higher speed to effectively predict the future for any given production line. Supervisors will reference output from the machine learning tools on these simulations to be able to predict the production trajectory and identify bottlenecks and aberrations hours before they occur, giving them time to make necessary adjustments.
Preparing for tomorrow’s needs
Process manufacturing organizations have recently discovered a multitude of reasons why key personnel may be limited. As fewer plant experts must cover a wider range of sites and processes, costs and delays rapidly increase. But while people are slow and costly to move, digital data is not. Life sciences organizations moving to a digital framework are building the foundation for remote manufacturing solutions that will drive more successful manufacturing and increased competitive advantage in the years to come. While we can’t know exactly what the future looks like, we do understand and have access to the technologies it is built upon and can begin taking steps today to implement those technologies to help ensure continued reliable access to critical treatments.
Bob Lenich is director of global life sciences at Emerson Automation Solutions. He is a life-long learner who stays engaged in new technology and organizational trends. In his 40 years in the industry, Bob continually aims to solve operating issues across the process industries to help Life Science manufacturing improve people’s lives. Bob has a BS in Chemical Engineering from Rose Hulman Institute of Technology and an MBA from the University of Texas.
Emerson Automation Solutions
About the Author
Bob Lenich
Director of global life sciences at Emerson Automation Solutions
Bob Lenich is director of global life sciences at Emerson Automation Solutions. He is a life-long learner who stays engaged in new technology and organizational trends. In his 40 years in the industry, Bob continually aims to solve operating issues across the process industries to help Life Science manufacturing improve people’s lives. Bob has a BS in Chemical Engineering from Rose Hulman Institute of Technology and an MBA from the University of Texas.