Nitrogen inerting for flammable and combustible liquids
By Nathan Bowser and Derek Miller
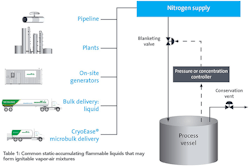
| Table 1: Common Static-Accumulating Flammable Liquids That May Form Ignitable Vapor-Air Mixtures |
| VM&P Naptha |
| Cyclohexane |
| n-Heptane |
| Benzene |
| Toluene |
| n-Hexane |
| Xylene |
| Ethyl Benzene |
| Styrene |
| Source: US CSB Case Study on Barton Solvents Explosion (No. 2007-06-I-KS) |
Even when required practices such as bonding and grounding are followed, flammable and combustible liquids can present the process industries with complex, persistent hazards.
NFPA Class II and Class III combustible liquids heated above flash point, nonconductive flammable and combustible liquids and special situations — such as switch loading — present significant risks equal to those presented by flammable liquids such as NFPA Class IB and IC and API Intermediate Vapor Pressure products.
A review of several industrial fires and explosions includes examples of hazards that were either unmitigated or improperly controlled. The examples demonstrate the role proper inerting applications can play in hazard protection.
It’s impossible to eliminate all possible ignition sources in all possible flammable environments, particularly given unexpected or off-spec operating conditions. Due to an abundance of variables that may cause an ignition — e.g., static electricity or other unforeseen spark generators — a focus on minimizing oxygen concentration by inerting is necessary in many cases.
The U.S. Chemical Safety Board (USCSB) notes how complex, persistently present and under-communicated flammable and combustible material hazards can be. It points out that standard protections such as bonding and grounding might not prevent accidents in cases involving nonconductive flammable liquids, which include many common materials (see Table 1).
Inerting practices mitigate these hazards, and are a benefit particularly where it is challenging or impractical to eliminate all ignition sources.
Done properly, inerting prevents fires and explosions above and beyond that of normal bonding and grounding and can even protect product quality.
Heated above flash point
Contributing factors in an explosion at a polyethylene wax-processing facility included combustible liquid heated above flash point, improper nitrogen-inerting system operation and improper pressure-vessel alterations. The explosion caused structural damage up to one-quarter of a mile away from the plant.
Processing polyethylene wax includes removing impurities, referred to as "rag," by heating rag wax to about 300 F using holding-tank steam piping. Post-accident testing by the CSB found the rag wax to have a flash point of 230 F, which classifies it as an NFPA Class IIIB combustible liquid. Heating the material above flash point generated sufficient vapor to produce a flammable environment, when mixed with a sufficient concentration of oxygen.
When failure of a weld on a pressurized holding tank led to spark generation, it initiated a flame that flashed back into the vessel, resulting in an internal deflagration and vessel failure.
The accident’s several root causes included improper rag-wax holding-vessel inerting. The facility generated nitrogen to prevent wax oxidation, as well as pneumatically transfer rag wax from the heated holding tanks. This same nitrogen system, properly used, could have saved the rag-wax holding vessel.
Unfortunately, the facility nitrogen generator pressure occasionally dropped below that required to transfer the rag wax. To maintain sufficient pressure to sustain operations, a compressed-air connection downstream of the nitrogen generator allowed mixing compressed air with the nitrogen stream.
Investigation determination
USCSB found this oxygen concentration, although lower than that of air (21 percent), adequate to support combustion. Given this oxygen concentration and the flammable rag-wax vapors in the holding vessels, two of the three elements necessary for a fire were present.
When the third element—an ignition source—manifested itself, the inevitable result was the accident described.
Many industrial accidents involve root causes that are occasionally unique, but often unforeseen because no one is consistently "overlooking."
Done correctly, inerting can prevent the escalations that lead to failures. Inerting the rag wax holding vessels may have prevented the flash-back into a vessel and the catastrophe that immediately ensued.
Something safer than the compressed air connection, such as liquid nitrogen backup or a different generator system, would have precluded attaining oxygen levels sufficient for combustion. Matching an inert gas supply system to the process utilization rates and flow patterns is important and can be accomplished either by an on-site generator, liquid supply, or combination of both.
Case Study CSB No. 2007-06-I-KS
Another noteworthy incident involving unexpected spark generation, ignition, and explosion occurred in a storage tank containing Varnish Makers’ and Painters’ naphtha. The naphtha’s flash point was found to be 58 F, and USCSB determined that at the handling temperature during the incident (approximately 77 F), there was likely an ignitable mixture in the tank-head space. Many materials, such as NFPA Class IB and IC flammable liquids, and API Intermediate Vapor Pressure Products, are capable of evolving flammable vapor-air mixtures at ambient conditions.
The naphtha involved also had a low electrical conductivity of 3 picosiemens per meter (pS/m), which allowed for a potentially hazardous accumulation of static electricity. Materials with conductivities below 100 pS/m are generally considered to be non-conductive. Many very common liquids fall within this category (see Table 1).
Processing these liquid materials through piping, tubing, or filters; splash filling; or stirring with splashing can cause them to accumulate static electricity. Decay of that static charge depends on the liquid’s conductivity and dielectric constant, so that even the liquid surface in contact with a bonded and grounded tank wall can have static potential.
The fire and explosion occurred while a tanker-trailer was loading naphtha into an above-ground storage tank. Witnesses confirm the tanker-trailer, pump, piping, and storage tank were all bonded and grounded at the time. The tank included a level float with a loose linkage that could interrupt grounding by slightly separating near the float.
What had transpired
USCSB determined that static charge accumulation occurred on the nonconductive liquid surface inside the tank and that a spark, likely generated by the loose linkage and intermittent loss of grounding, ignited the flammable vapor-air mixture in the tank head space. The result was a fire and explosion that destroyed the tank farm and sent eleven residents and one firefighter for medical treatment.
A key investigation finding was that the tank had an ignitable vapor-air mixture in its head space. Proper inerting techniques could have mitigated this hazard by reducing oxygen concentration to below ignition-support levels. As previously mentioned, bonding and grounding typically employed to mitigate static- charge accumulation hazard but, in many cases, it may not be enough.
Although the USCSB determined the loose linkage was the most likely spark location, a spark from a brush discharge could not be ruled out. According to Britton, brush discharges can occur even when equipment is properly bonded and grounded during loading and unloading operations. In this case, two legs of the fire triangle — fuel and an ignition source — can be present, and a focus on eliminating the oxygen content is needed. The USCSB specifically noted in this incident investigation that extra precautions should be taken by companies that store, transfer, and handle nonconductive flammable liquids due to these risks.
For those companies, inerting solutions exist to protect against the unexpected. Storage tanks can be blanketed using a form of concentration or pressure control, which can be more economical than blanketing via continuous purging. Based on the material’s limiting oxygen concentration, process operating pattern and flow rate needs, an inert-gas purity and supply mode (or combination of supply modes) can be recommended. Nitrogen is the most commonly used inerting gas, but carbon dioxide or argon can be used where nitrogen is not appropriate.
Those special cases
Several additional, special cases present significant flammability hazards. Examples include off-gassing of abnormal volatile components in a low-vapor pressure liquid; fine droplets, mist, or foam on the liquid surface; processing liquids at non-standard temperatures or pressures capable of creating ignitable vapors; or practices such as switch loading.
"Switch loading," as defined by API 2003, refers to the practice of loading a low-vapor pressure liquid into a container, e.g., trailer, tank or vessel, which previously contained a high or intermediate vapor pressure product. The hazard exists because the head space, which is often above the upper-flammability limit with the high or intermediate vapor-pressure product, may drop into the flammable range while loading the low-vapor pressure product.
In the event of a spark or static discharge from liquid loading, the flammable mixture in the head space could ignite, resulting in fire, explosion or both. Switch loading of nonconductive liquids increases this risk since any static charge build-up will not be bled off as easily via bonding and grounding. This practice has resulted in numerous fires in industry. Sufficient vessel inerting prior to loading the low-vapor pressure liquid can protect against these hazards by eliminating the oxygen content as well as the ignitable vapors from the previous liquid.
Final words
Combustible liquids heated above their flash point, nonconductive flammable and combustible liquids, and special situations such as switch loading present significant risks that warrant additional precautions. Inerting is a powerful and flexible solution that can mitigate fires and explosions for these situations. Inert gases can present the risk of asphyxiation if not used properly. Therefore safe handling procedures must be understood and followed. In addition, materials such as ethylene oxide contain oxygen in the molecule that can yield flammability, without additional oxygen. Therefore, proper design of an inerting system and its supply mode are based on the facility, processes, and hazards at hand. Experts in applying inert gas solutions are essential resources when designing an application and can help facilities improve their understanding as well as their process safety.