How to eliminate clamp galling: The advanced strategies and clamps that can keep your processes operational
Pharmaceutical applications require hygienic environments. Companies involved in pharmaceutical production must ensure tight controls on product purity through every stage of the development process, from manufacturing and quality control to packaging and shipping. Due to the tight restrictions that the FDA and other regulatory agencies place on these companies, a single contaminated batch of a standard high-performing drug like heart medication or insulin can cost hundreds of thousands — if not millions — of dollars in lost revenue. Extrapolate that out for the most expensive drugs on the market, and that cost can skyrocket to insurmountable levels.
That is why every piece in the complex puzzle of vessels, piping, tubing, valves and pumps that make up pharmaceutical processing systems need to be designed with hygienic processes in mind. Above all else, this means reliable hygienic connections. Batch process facilities need long-lasting clamps that not only provide consistent connections to prevent leaks, but that can also be easily removed for maintenance, washdowns and change-outs. Hygienic clamps must be able to do all of this while still operating in high-pressure and high-temperature environments.
With decades of experience helping leading pharmaceutical manufacturers maintain hygienic processes, the team at LJ Star is dedicated to keeping pharmaceutical companies up and running. While quality clamps are essential in these applications, many clamps in the past were susceptible to galling. Galling refers to a type of fused wear in which a clamp’s bolt and nut become bound or locked together, which can halt operations and even risk process contamination in the most severe cases. That is why LJ Star has set out to define the mechanisms behind galling — and how to prevent it.
Is stainless steel enough?
The simple solution would seem to be using stainless steel sanitary clamps. Stainless steel is generally rather durable compared to other metals and can withstand the harsh chemicals and caustic washdowns used to sanitize pharmaceutical processes. Stainless steel also maintains its mechanical strength and integrity when operating under high temperatures and pressures, so it would seem perfect for the pharmaceutical industry.
While all of this is true, there is an issue with using your typical stainless steel for sanitary clamps. When both the bolts and the nuts of a clamp are made of the same grade of stainless steel, it makes them more susceptible to galling. Also referred to as thread galling, galling causes the threads of the nut and the bolt to become fused together, making it extremely difficult — if not downright impossible — to open and remove the clamp. This prevents the necessary inspections and servicing that is FDA-mandated in the pharmaceutical industry. With many batch operations requiring personnel to remove and reassemble these connections with each new batch, this is not just a major hindrance to productivity, but a total process killer. To better understand how typical stainless steel stacks up against other types on wear and galling, you can find the wear compatibility of stainless-steel couples in Table 1 below:
The science behind galling
Galling is a type of abrasive wear that can happen when two metals rub together. Here, this is referring to the nut and bolt of the clamp, which is why it is also called thread galling. When a nut and bolt connection is over-torqued, either manually or mechanically, the separate pieces of metal can fuse together or bind to each other. This means that they are literally “cold-welded” together in what is known as a solid-phase welding process.
Loads must be sufficient during relative motion between the nut and the bolt for there to be a transfer of material from one part to the other. With the right combination of higher loads and stress, along with poor lubrication, stronger bonds can form over a larger surface area, increasing the severity of the galling. Since it can result in the immediate seizure of metal components, severe galling is anything but a temporary maintenance difficulty.
While galling can feasibly occur in any metal that is rubbing up against another metal, softer metals and metal connections of the same type are more prone to galling. Highly ductile materials and those that possess low work-hardening rates are also more susceptible to galling. Austenitic stainless steels, typically known for their corrosion resistance, also show a more significant tendency to gall under certain instances. These conditions and other risk factors for galling include:
- Dirty or damaged threads
- Installing too quickly or under a load
- Not applying lubricants
- Using locking fasteners
Since galling is essentially the metals breaking down at the surfaces where they meet, metals that are more susceptible to wear and corrosion have a higher chance of galling. The more particulate that is generated due to wear and corrosion, the more severe the galling.
While stainless steel is naturally very resistant to corrosion, this is largely due to the layer of protective oxide film over the metal. With the friction required to turn a nut around a bolt, the excess heat causes the protective layer to wear off, leaving the softer and more reactive metal exposed, and thus significantly increasing the chance of galling. Once that protective layer is gone, the threads between the nut and bolt will continue to generate heat and undergo increased pressure as the connection is tightened, causing the metals to “stick” together.
Aside from the mechanical onset of galling through the assembly and disassembly of a clamp, galling can also occur as a result of the strict cleaning procedures that are standard in pharmaceutical plants. If you use a parts washer to clean a clamp prior to installation, it can leave behind trace amounts of the sodium hydroxide that is used in clean-in-place (CIP) solutions. Once a solution dries, those trace amounts of chemicals can build up in the grooves of the clamp’s threaded parts, adding to the risk factors of galling and further binding the parts together.
Why galling is so dangerous
In minor cases of galling, the fasteners can still be removed by applying lubricant and using extracting tools. However, in more severe cases, the clamps will need to be cut off, forcing the piping below it to be removed. The trouble here is that it is often not easy to identify if galling is occurring until it is too late.
While the dangers of not being able to remove a clamp can quickly become a costly problem for pharmaceutical processors, what is even worse is that this can lead to product contamination. Galled clamps that are over-torqued will exert excess pressure on the connection, which may lead to gasket intrusions into the connection. This interferes with the flow of process materials, allowing particles to collect around the intrusion. An over-torqued clamp can even lead to tiny pieces of the gasket shearing off and being carried away, contaminating whatever batch of product is being processed at that time.
If even the tiniest amount of contaminant makes its way into a batch of products, the entire batch needs to be thrown out. Not only does the instigating clamp need to be replaced, but all other process vessels must also be taken apart for inspection and cleaning. There is no way around this. Once a clamp galls, if it causes product contamination, it is game over. That is why the chance of galling needs to be eliminated from both a strategic and technical level.
Using better processes to avoid galling
While much of the galling issue stems from a problem with parts and materials, there are advanced installation and maintenance strategies that you can use to help prevent galling.
In general, using fastener lubrication is one of the simplest strategies to avoid galling. Anti-seize solutions can help prevent the mechanisms of galling, no matter what parts or materials you are using. However, using added lubricants and solutions is often not an option in strict pharmaceutical environments, which is why other strategies are required.
Since friction is what causes fasteners to get hotter and hotter during installation — ultimately leading to galling as protective layers wear away — slowing down installation speeds will cause the created heat to dissipate even more. This is not a foolproof strategy, and can be its own issue when it comes to keeping downtime and maintenance durations to a minimum, but it can be helpful when applicable.
- Other strategies to help prevent galling include:
- Making sure that fastener threads are not already damaged or compromised before installation
- Keeping fasteners in a cool, controlled environment
- Making sure that there is no grime, dirt or debris in the threading of a fastener
Of course, to prevent galling, you will also want to mitigate the torque applied to your fasteners. To avoid overtightening a clamp, process technicians should always use approved torque tools. For example, using a torque wrench instead of a regular wrench can help avoid over-torquing a nut, which causes wear on its threads.
Solving the galling issue with better clamps
While using advanced strategies can certainly help to prevent galling, you can only do so much with inferior parts. Available on market today is a novel new solution to help prevent galling in pharmaceutical applications: the anti-galling clamp.
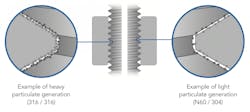
The power behind the anti-galling clamp comes from a combination of design and materials. Rather than using typical stainless steel, these clamps use Nitronic 60 (Alloy 218, UNS S21800), which is known for its excellent galling resistance, even in high-temperature operations. Adding 4% silicon and 8% manganese to the steel inhibit galling, wear and fretting. Since Nitronic 60 is more resistant to the mechanism of wear, there is less particulate generated by on/off cycles, shown in Figure 2.
Spotlight on Nitronic 60
Nitronic 60 (Alloy 218, UNS S21800) provides excellent galling resistance, even when used in applications with elevated temperatures or high-impact operations. By adding 4% silicon and 8% manganese, this alloy becomes resistant to wear, galling and fretting. Nitronic 60 maintains decent strength up to a temperature of 1,800°F while still having an oxidation resistance similar to 309 stainless steel. It offers a corrosion resistance between that of 304 and 316 stainless, yielding a strength twice that of either 304 or 316.
By generating less particulate, not only do these Nitronic 60 clamps have less of a risk of galling, but also less of a chance that any batch-ruining particulate could make its way into your product. Other advantages of the anti-galling clamp include:
- Significantly decreased likelihood of clamp galling
- Reduced particulate generation
- Increased service life
- Higher resistance to CIP cleaning solution residue
- Increased torque value consistencies after CIP
With these advantages, using anti-galling Clamps can help to optimize your processes. Having an anti-galling clamp installed will help give you peace of mind knowing that your hygienic clamps aren’t compromising your process lines.
Jeremy Sheldon is National Sales Manager, Life Sciences for LJ Star. He joined the company in 2019 and has spent his career with a variety of biomedical and pharmaceutical companies. Jeremy earned a bachelor’s degree in biology from The University of Akron.
About the Author
Jeremy Sheldon
National Sales Manager, Life Sciences, LJ Star
Jeremy Sheldon is National Sales Manager, Life Sciences for LJ Star. He joined the company in 2019 and has spent his career with a variety of biomedical and pharmaceutical companies. Jeremy earned a bachelor’s degree in biology from The University of Akron.