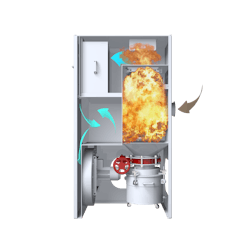
Dust collection in continuous solid dose pharmaceutical manufacturing processes
Unlike batch production of solid dose pharmaceuticals, in which a predetermined weight of ingredients is processed before being discharged as finished product, continuous solid dose manufacturing simultaneously charges and discharges ingredients and finished product. Uninterrupted dust collection is critical in continuous manufacturing operations because production can run 24 hours per day, 7 days per week.
Dust collectors extract potentially toxic and combustible dusts from pharmaceutical processes including coating, tableting, blending, drying and packaging. They use filter cartridges to remove dust particles from the contaminated process air that may pose a risk to the health of workers. Dust particles in the air can also cause combustible dust explosions and fires, damage products and production equipment, and cause cross contamination.
Here are some proven measures that will help your dust collection system meet this challenge around the clock.
Choose the right size dust collector
Continuous solid dose manufacturing usually requires smaller processing equipment and facilities, which tend to be faster and less expensive to design, build and install. The processes can be simpler and more efficient to run and maintain, saving time and money. Compact, fully integrated equipment is required to meet these demands without compromising on product quality or operational safety.
Small process machines and reduced floor space require small ancillary equipment. This includes dust collectors and the cartridge-style filters that dry dust applications typically use because they have a greater surface area and smaller footprint than bag filters. Dust collectors typically need to incorporate a HEPA filter and a fan, which can take up valuable space on a production floor or suite. The solution is to install a fully integrated compact dust collection system.
Enable continuous operation via self-cleaning filter system
Dust collectors can run continuously when they clean their own filters using reverse pulse cleaning. This type of cleaning system uses compressed air to remove dust on the filter cartridges that is restricting the airflow and raising the dust collector’s differential pressure (dP). Nozzles direct pulses of compressed air into the inside of the filter cartridges to eject the dust from the surface of the media.
The most efficient way to operate a reverse pulse cleaning system is to use a controller that reads the dP across the filters. That way, the cleaning system initiates automatically when the high set dP point is reached and pulse cleans down to the low set point, which returns it to a stable level.
The ejected dust is stored in a suitable receptacle where it can be emptied when necessary without having to shut down the dust collector. However, it is possible for the pulse cleaning process to cause pressure disturbances in the dirty air ducting and the process machine to which it is connected. That is why it is important to use proactive strategies to prevent these disturbances such as reducing the compressed air pressure, using segmented filter designs, scheduling periodic off-line cleaning and adding automatic dampers. All these techniques can be designed into the system to help prevent pressure issues that can negatively affect the connected processing machine.
Improve energy efficiency
In continuous manufacturing, energy efficiency is a primary consideration for the design of all ancillary equipment, including the dust collectors. Dust collectors consume energy in two main areas: the fan and the pulse cleaning system. The fan produces the negative pressure to effectively remove the dust that is generated. Dust collectors connected to well-balanced extract ducting minimize the fan’s power consumption. Using variable frequency drives and high-efficiency motors can also reduce energy consumption.
The second main area of energy consumption is the filter cleaning system. Compressed air is expensive to generate, so the more efficient the filter cleaning process, the lower the average dP and compressed air consumption will be. Therefore, it is important to consider the following when selecting the most energy-efficient dust collector for the application: dust release characteristics of the filter media, design of the filter cartridges, design of the pulse cleaning mechanism, and how they all work in concert to reduce energy consumption.
Use the right safety features
Before implementing a continuous process, pharmaceutical manufacturers must evaluate potential health and safety risks. With dust collection, the main hazards involve potentially exposing workers to harmful dusts, cross-contaminating other products, and generating a fire or dust explosion from combustible dust. A well-designed and properly maintained dust collection system can remove airborne dust from the processing workspace and provide safeguards against combustible dust explosions and employee exposure to hazardous dusts.
Removing collected dust, changing filters and performing maintenance work on the system can expose workers to hazardous dusts. Bag-in/bag-out containment systems and continuous liner technology reduce these risks. These types of systems use a combination of physical barriers and operator techniques to isolate the user from the contents of the dust collector. Also look for a safe-change system that is ergonomically designed and easy to use to help workers perform maintenance carefully and consistently.
The surrogate testing process can help validate the effectiveness of dust isolation and containment equipment in reducing potential exposures and contamination risks. Surrogate testing assesses risks by simulating the hazardous material with a substitute compound. The test compound simulates the hazardous material in the formulation that the solid dose equipment will handle or process. The test compound is manipulated to simulate operations for either a typical processing workday or a worst-case scenario. Surrogate testing most often occurs under the guidance of a manufacturer’s health and safety team in conjunction with experts from an independent testing facility. This type of testing is typically conducted by the equipment supplier, so be sure to request its testing results when specifying containment options for dust collectors.
Regarding explosion protection standards, processing personnel can effectively manage fire and explosion risks if they fully understand the characteristics of the manufacturing process and dust explosion properties of the materials being processed. Dust explosion characteristics are known for many products used in manufacturing processes, and this information, even for proprietary products, is required under National Fire Protection Association (NFPA) standards. When this information is not available, the manufacturer can contract a specialist lab to conduct explosivity testing to determine values such as the Kst (rate of explosion), Pmax (maximum explosion pressure) and MIE (minimum ignition energy). This information is required to select the most appropriate explosion protection equipment for the dust collection system. With this knowledge, the dust collector equipment supplier can properly design a safe, NFPA-compliant system for the specific continuous manufacturing process.
For processes with small air volumes, manufacturers can sometimes avoid specifying expensive explosion protection equipment by selecting an advanced dust collection system that has documented, performance-based testing showing that it can safely contain dust explosions. The cost savings can be significant with compact dust collectors, particularly with respect to installation and ongoing maintenance costs.
Summary
Dust control can be a difficult challenge in solid dose pharmaceutical processing, and it is imperative for manufacturers to keep their workers and facilities protected while maintaining product quality. Continuous manufacturing adds the extra complexity of maintaining this level of safety and productivity around the clock. When properly designed, a dust collection system can match the demands for performance, safety and reliability.
David Steil is pharmaceutical market manager at Camfil Air Pollution Control (APC), where he has worked for the past 13 years. A graduate of Indiana University of Pennsylvania with a Bachelor of Science degree in safety and industrial hygiene, David spent more than 12 years with Wyeth Pharmaceuticals as a member of its corporate Environment, Health and Safety (EHS) group. He joined Camfil APC in 2007 as sales and technical support liaison serving the pharmaceutical market. He then moved into the role of biotech/pharmaceutical market manager before his current position. David is a member of the International Society for Pharmaceutical Engineering (ISPE) and the American Industrial Hygiene Association (AIHA). Camfil APC is a global manufacturer of dust, fume and mist collection equipment for challenging industrial applications, with production facilities around the world including the Americas, Europe and Southeast Asia.
Camfil Air Pollution Control
About the Author
David Steil
David Steil is pharmaceutical market manager at Camfil Air Pollution Control (APC), where he has worked for the past 13 years. A graduate of Indiana University of Pennsylvania with a Bachelor of Science degree in safety and industrial hygiene, David spent more than 12 years with Wyeth Pharmaceuticals as a member of its corporate Environment, Health and Safety (EHS) group. He joined Camfil APC in 2007 as sales and technical support liaison serving the pharmaceutical market. He then moved into the role of biotech/pharmaceutical market manager before his current position. David is a member of the International Society for Pharmaceutical Engineering (ISPE) and the American Industrial Hygiene Association (AIHA).